Chemical reactions can produce new elements. C A nuclear reaction is unaffected by the chemical state of the atoms involved.

Dna Vs Rna 5 Key Differences And Comparison Technology Networks
Firing a neutron at the nucleus of the atom.
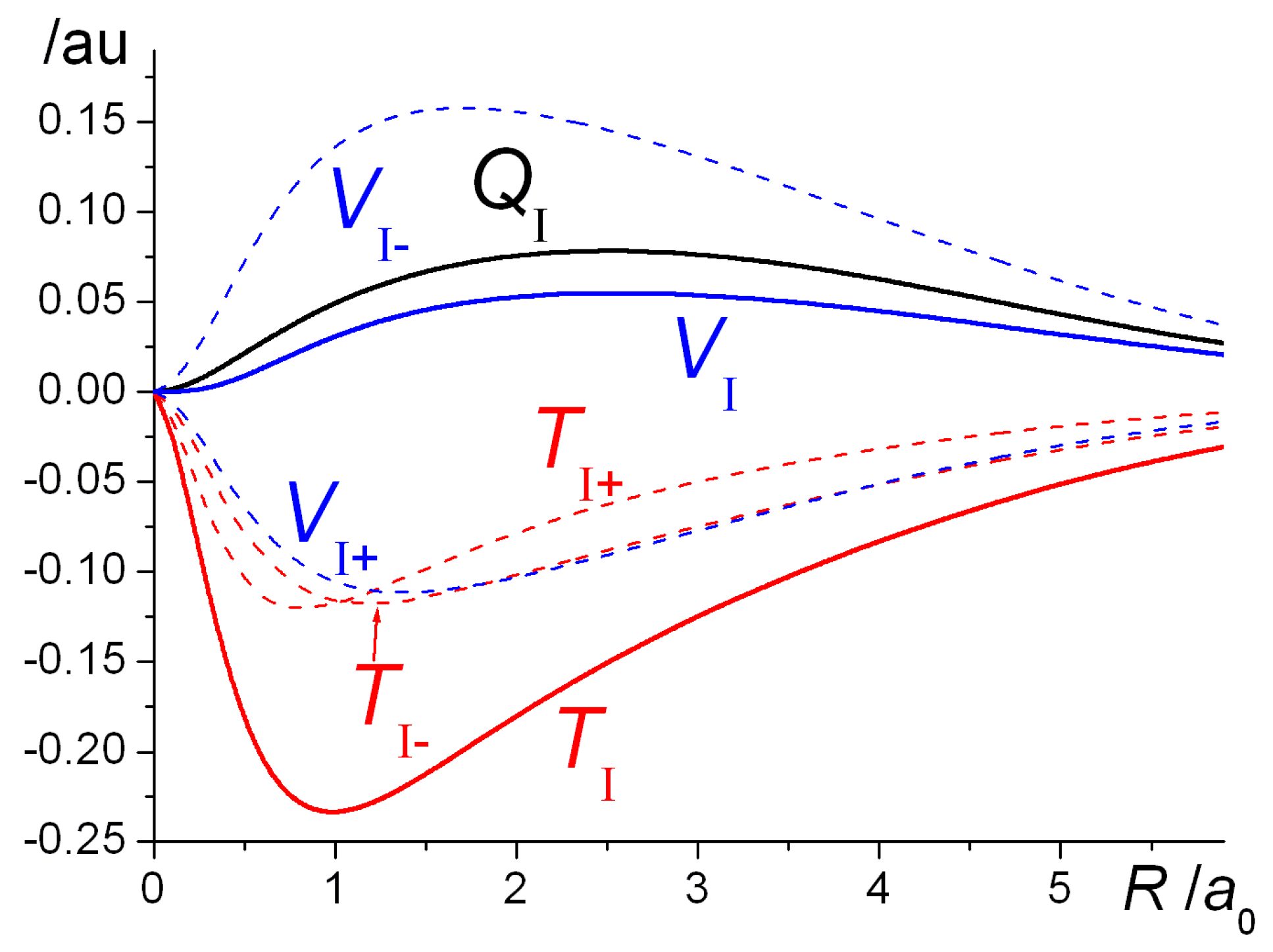
. Chemical reactions can produce new elements. The nucleus of an atom participates in a chemical reaction. A change that affects the nucleus of an atom.
An example is an atomic bomb. Chemical reactions can produce new elements. Identify the number of atoms on the reactant side of the chemical equation.
Which statements describing chemical and nuclear reactions are true. All of the statements about nuclear reactions are true except a Nuclear reactions involve changes in the nucleus of an atom. Nuclear reactions do not require conservation of mass.
Nuclear reactions require heat or an input of energy to OCcur. Nuclear reactions involve substantially greater energy changes than ordinary chemical reactions. Which of the following reactions is a fission reaction.
In your own words describe the purpose of using a chemical equation. 3 Nuclear reactions can produce. Which of the following statements regarding nuclear reactions is false.
Which statement describing chemical and nuclear reactions are true. The nucleus is unaffected. Isotopes behave the same way in nuclear reactions.
Which statement describes the differences between chemical reactions and nuclear decay rates. The amount of energy in the bonds of the reactants plus any energy absorbed equals the amount of energy in the bonds of the products plus any energy _____. Which of the following statements is true about a chemical reaction.
Nuclear reactions involve much higher energy changes when compared to chemical reactions. Fission produces energy while fusion requires energy. After the reaction he records a mass of 43 g for the zinc sulfate solution and copper produced by.
C In a chemical reaction mass is conserved. Isotopes of the same element behave differently in a nuclear reaction. There are 2 Fe iron atoms.
Which statements describing chemical and nuclear reactions are true. Nuclear decay follows first order kinetics. Which of the following processes can be used to start a nuclear fission reaction in certain atoms.
There are H hydrogen atoms. The rate of nuclear decay is not temperature dependent. Which statements describe nuclear reactions.
Chemical reactions involve the rearrangement of electrons. Nuclear reactions involve the nuclei of atoms. Which of the following statements about nuclear reactions is true.
D Nuclear reactions of the same element vary according to which isotope is involved. Fe2O3 3H2 2Fe 3H2O. A It involves changing the ways the atoms are grouped.
23994 Pu 10 n -- 14458 Ce 9436 Kr 2 10 n. Nuclear reactions produce only different compounds. B The rate of a nuclear reaction is increased by the addition of a catalyst.
Nuclear reactions require heat or an input of energy to occur. 1 The electrons of an atom participates in a chemical reaction. Select all that apply.
A small amount of mass changes into energy. Billy Bob combines zinc Zn and a copper II sulfate solution CuSO4 to produce a zinc sulfate solution ZnSO4 and copper Cu. Chemical reactions involve the rearrangement of electrons.
The nucleus of an atom participates in a chemical reaction. The products of nuclear reactions are lighter than the reactants. B In a chemical reaction atoms are neither created nor destroyed.
2 Chemical reactions involve the rearrangement of electrons because there is transfer loss gain and sharing of electrons in chemical reactions. Check all that apply. The same number of each kind of atom appear in the reactants and products.
Chemical reactions involve the rearrangement of electrons and 5. The nucleus of an atom participates in a chemical reaction. D all of the above E none of the above.
In progress 0 Chemistry Mít Mít 7 months 2021-07-28T1031170000 2021-07-28T1031170000 1 Answers 6 views 0. Before the reaction he records a beginning mass of. Chemical reactions involve the rearrangement of electrons.
The nucleus of a larger atom breaks into two smaller nuclei. 43 grams g of the zinc and copper II sulfate solution. The products of nuclear reactions are heavier than the reactants.
Nuclear reactions require heat or an input of energy to occur. Chemical equations show how chemicals interact when a reaction occurs. Chemical reaction rates vary but nuclear decay rates are constant.
Which statements describing chemical and nuclear reactions are true. Nuclear reactions involve the electrons of atoms. Which statements describing chemical and nuclear reactions are true.
There are O oxygen atoms.
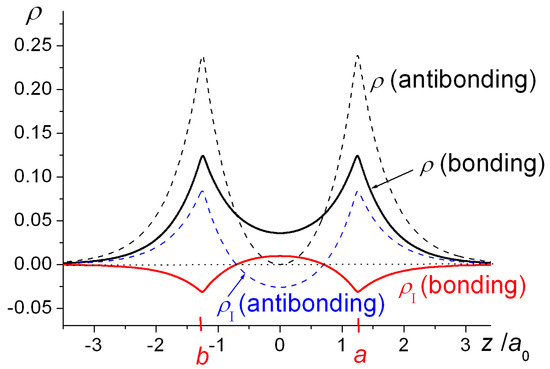
Molecules Free Full Text The Basics Of Covalent Bonding In Terms Of Energy And Dynamics Html

Molecules Free Full Text The Basics Of Covalent Bonding In Terms Of Energy And Dynamics Html

Identify Whether The Following Statements Describe Fusion Fission Or Both Fission And Fusion A A Nuclear Reaction That Releases Enormous Amounts Of Energy B A Large Nucleus Breaks Apart To Form Smaller
0 Comments